Expand your
possibilities.
Learn more*Posed by model, not actual patient
The OPERA-01 trial will test how safe and effective the investigational drug palazestrant (OP-1250) is compared to one of the available standard-of-care medications. This phase 3 study is for people with advanced and/or metastatic estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-) breast cancer that has progressed after receiving CDK4/6 inhibitor medicines in combination with an endocrine (hormone) therapy.
About 80% of all breast cancers are hormone receptor-positive (HR+). Most HR+ breast cancers are estrogen-receptor positive (ER+). Therefore, sometimes ER+ breast cancer is referred to as HR+ breast cancer.
Learn moreAbout the trial
What is the OPERA-01 trial?
The OPERA-01 Phase 3 trial will evaluate how safe and effective the investigational drug palazestrant is compared to one of the available standard-of-care medications in adults with ER+/HER2- advanced and/or metastatic breast cancer that has progressed after receiving CDK4/6 inhibitor medicines in combination with an endocrine therapy.
What will happen in this clinical trial?
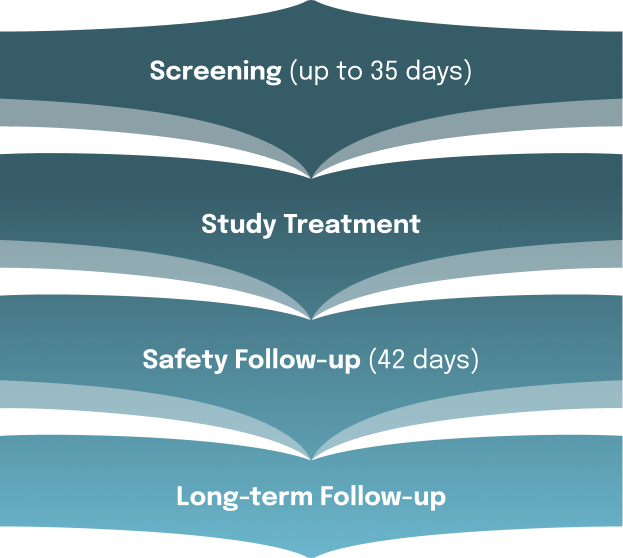
If you are eligible to enroll in this trial, you will be randomly assigned (by chance) to receive the study drug (palazestrant) or a standard-of-care endocrine therapy for advanced breast cancer. You and your study doctor will be aware of your study group assignment. There is no placebo being used in the trial.
Palazestrant oral tablets will be taken once daily. Standard-of-care endocrine therapy will be administered either as tablets taken once daily or as injections (every four weeks, except twice in the first month).
Other tests including medical examinations and laboratory assessments will be performed at the regular visits.
How long will the OPERA-01 trial last?
Your time in the trial will vary. You should expect to receive palazestrant or standard-of-care endocrine therapy until you or your doctor decide to stop participation.
Who is the ideal candidate for OPERA-01?
After consultation with their own physician, potential study subjects who meet the following criteria may be eligible to participate in the OPERA-01 trial:
- Adult women or men, at least 18 years old
- Diagnosis of ER+/HER2- advanced and/or metastatic breast cancer that has progressed after prior
endocrine therapy - Previously received a CDK4/6 inhibitor medicine in combination with an endocrine therapy
- Have not previously received chemotherapy
- Female participants can be pre-, peri-, or postmenopausal
Please note, this is not a complete list of study requirements. The study coordinator will review the full list of requirements with each study subject. One can find more information about the OPERA-01 trial at clinicaltrials.gov (NCT06016738).
Learn moreYour questions answered
Why is the OPERA-01 trial important?
Breast cancer is one of the most prevalent cancers worldwide, with more than two million new diagnoses globally each year. Trials like OPERA-01 help researchers better understand breast cancer and look for drugs that could help more people with advanced or metastatic disease.
What are the goals of the OPERA-01 trial?
The main goals are to evaluate how safe and effective an oral study drug palazestrant is compared to one of the available standard-of-care medications in adults with ER+/HER2- advanced and/or metastatic breast cancer that has progressed after receiving CDK4/6 inhibitor medicines in combination with an endocrine therapy.
What is a clinical trial?
A clinical trial is a research study involving volunteers (also called participants) that is intended to add to medical knowledge. Clinical trials are important to help improve how advanced breast cancer is treated today and discover new solutions for the future. These advances are only possible thanks to volunteer participation.
What is a study drug?
A study drug (also called an investigational drug) is a substance that is being tested in a clinical trial and has not been approved by regulatory agencies, such as the United States (US) Food and Drug Administration (FDA), European Medicines Agency (EMA), or others. Every study drug is reviewed by an ethics committee (or institutional review board) for testing in people, and the study doctors and healthcare professionals must follow strict rules set by the FDA and other regulatory agencies.
What are the costs to take part in the OPERA-01 trial?
You do not have to pay for the study drugs or any tests that are part of this trial. You may be reimbursed for the reasonable costs associated with travel (if needed) to participate in this trial.
What risks are involved in trial participation?
There are risks involved with any clinical trial. A member of the trial team will review the risks with you, and you will be closely monitored throughout the trial.
What if I change my mind or decide to stop participating?
Taking part in a trial is voluntary. Participants are free to leave the trial at any time.
What happens when the trial ends?
After a clinical trial is completed, the researchers carefully examine information collected during the trial before making decisions about the meaning of the findings and about the need for further research. The outcome of the trial will be made publicly available.
How can I learn more?
For more information about the OPERA-01 trial, please contact us at [email protected] or visit clinicaltrials.gov.
Thank you for your interest in the OPERA-01 trial.
If you would like to learn more about this trial, please contact us at [email protected] or visit clinicaltrials.gov.
Trial site locations
Visit clinicaltrials.gov to see OPERA-01 trial site locations near you.
